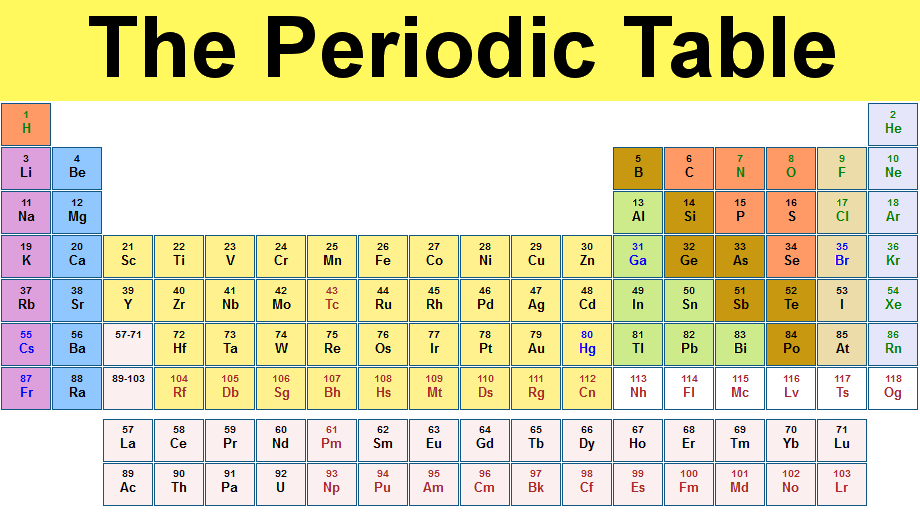
| Group | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Period | |||||||||||||||||||
| 1 |
1
1.008
|
2
4.0026
| |||||||||||||||||
| 2 |
3
6.94
|
4
9.0122
|
5
10.81
|
6
12.011
|
7
14.007
|
8
15.999
|
9
18.998
|
10
20.180
| |||||||||||
| 3 |
11
22.990
|
12
24.305
|
13
26.982
|
14
28.085
|
15
30.974
|
16
32.06
|
17
35.45
|
18
39.948
| |||||||||||
| 4 |
19
39.098
|
20
40.078
|
21
44.956
|
22
47.867
|
23
50.942
|
24
51.996
|
25
54.938
|
26
55.845
|
27
58.933
|
28
58.693
|
29
63.546
|
30
65.38
|
31
69.723
|
32
72.63
|
33
74.922
|
34
78.96
|
35
79.904
|
36
83.798
| |
| 5 |
37
85.468
|
38
87.62
|
39
88.906
|
40
91.224
|
41
92.906
|
42
95.96
|
43
[97.91]
|
44
101.07
|
45
102.91
|
46
106.42
|
47
107.87
|
48
112.41
|
49
114.82
|
50
118.71
|
51
121.76
|
52
127.60
|
53
126.90
|
54
131.29
| |
| 6 |
55
132.91
|
56
137.33
| * |
71
174.97
|
72
178.49
|
73
180.95
|
74
183.84
|
75
186.21
|
76
190.23
|
77
192.22
|
78
195.08
|
79
196.97
|
80
200.59
|
81
204.38
|
82
207.2
|
83
208.98
|
84
[208.98]
|
85
[209.99]
|
86
[222.02]
|
| 7 |
87
[223.02]
|
88
[226.03]
| ** |
103
[262.11]
|
104
[265.12]
|
105
[268.13]
|
106
[271.13]
|
107
[270]
|
108
[277.15]
|
109
[276.15]
|
110
[281.16]
|
111
[280.16]
|
112
[285.17]
|
113
[284.18]
|
114
[289.19]
|
115
[288.19]
|
116
[293]
|
117
[294]
|
118
[294]
|
| *Lanthanoids | * |
57
138.91
|
58
140.12
|
59
140.91
|
60
144.24
|
61
[144.91]
|
62
150.36
|
63
151.96
|
64
157.25
|
65
158.93
|
66
162.50
|
67
164.93
|
68
167.26
|
69
168.93
|
70
173.05
| ||||
| **Actinoids | ** |
89
[227.03]
|
90
232.04
|
91
231.04
|
92
238.03
|
93
[237.05]
|
94
[244.06]
|
95
[243.06]
|
96
[247.07]
|
97
[247.07]
|
98
[251.08]
|
99
[252.08]
|
100
[257.10]
|
101
[258.10]
|
102
[259.10]
| ||||
Latest news
On August 12th experiments involving zinc ions travelling at 10% of the speed of light colliding with a thin bismuth layer apparently produced a very heavy ion followed by a chain of six consecutive alpha decays identified as products of an isotope of element 113 278>Uut - see new evidence for elements 113.Confirmation of the discoveries of and name proposals for elements 114 (flerovium) and 116 (livermorium)
A news reports from IUPAC (more about flerovium and livermorium) indicates the confirmation of the discoveries of elements 114 and 116: Discovery of the Elements with Atomic Number 114 and 116. Proposals by the discoverers for the names of the two elements have now announced as:- element 114: Flerovium (Fl) after the physicist Georgiy Flerov. Georgiy N. Flerov (1913-1990) was a renowned physicist who discovered the spontaneous fission of uranium and was a pioneer in heavy-ion physics.
- element 116: Livermorium (Lv), after the Livermore laboratories. A group of researchers from the Laboratory, along with scientists at the Flerov Laboratory of Nuclear Reactions, participated in the work carried out in Dubna on the synthesis of superheavy elements, including element 116.
Buy Periodic Table posters
Element 117 discovered?
A paper just published (5 April 2010) in Physical Review Letters by Yu. Ts. Oganessian and others claims the synthesis of a new element with atomic number 117. The abstract states "The discovery of a new chemical element with atomic number Z=117 is reported. The isotopes 293117 and 294117 were produced in fusion reactions between 48Ca and 249Bk. Decay chains involving eleven new nuclei were identified by means of the Dubna Gas Filled Recoil Separator. The measured decay properties show a strong rise of stability for heavier isotopes with Z>=111, validating the concept of the long sought island of enhanced stability for super-heavy nuclei." Read more
11
22.990
12
24.305
13
26.982
14
28.085
15
30.974
16
32.06
17
35.45
18
39.948
11
22.990
12
24.305
13
26.982
14
28.085
15
30.974
16
32.06
17
35.45
18
39.948


ไม่มีความคิดเห็น:
แสดงความคิดเห็น